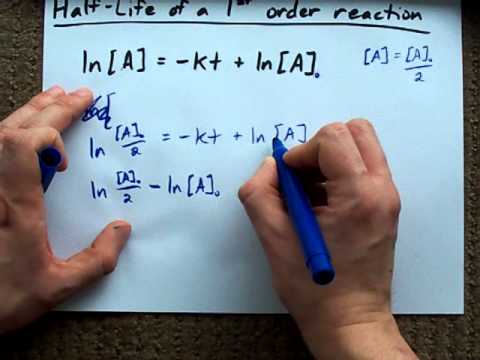
Half-Life of a First-Order Reaction (Derivation) - YouTube
Mar 13, 2013 ... What is the expression for Half-Life of a First Order Reaction?Here, I derive it from the integrated rate law.The answer is t = ln 2 / kAsk me ...
Half Lite First Order : Useful Links
Answer to - w ysor rurrure proud loodcrive Stance have a characteristic half-lite. From first order kinetics the half life, Iva, i...
search-thumbnail-A A reaction has a half lite of 10 minutes. $A$ A reaction has $ a$ ...
Answer to s ^ - 1 what is its half - lite Question 1 ( point the rate constant for a first - order reaction is 0.05012 ? Please in...
$A$ A reaction has $a$ half $lite$ of $10$ minutes. Calculate the rate constant for the first order reaction. 1st-6th grade. Chemistry. Solution. answer-user-profile - ...
$20 Off + FREE Delivery on your first order!* Enter code SAVE20 at checkout ...
This Black Friday is your chance to upgrade from Pluckers Club Lite or join the Pluckers Club for the first time! Pluckers Club memberships are now half-price for ...
The half-life of a reaction is the time required for the concentration of a reactant to decrease to one half of its initial concentration. All first-order reactions have ...
Take 15% off your first order over $19.98 with code: BULKLIFE.
Special Golf Achievements: All time low 18-hole score: 60; Most consecutive birdies: 7; Career "Hole-in-One": 20; 92 victories (62 US Tour, 18 foreign/ international, ...